Formation of Drusen: NIH Study Reveals Cellular Pathology of Dry AMD
A report by scientists at the National Institutes of Health (NIH) and Johns Hopkins University, Baltimore, details how alterations in a factor called AKT2 affects the function of organelles called lysosomes and results in the production of deposits in the retina called drusen, a hallmark sign of dry age-related macular degeneration (AMD)
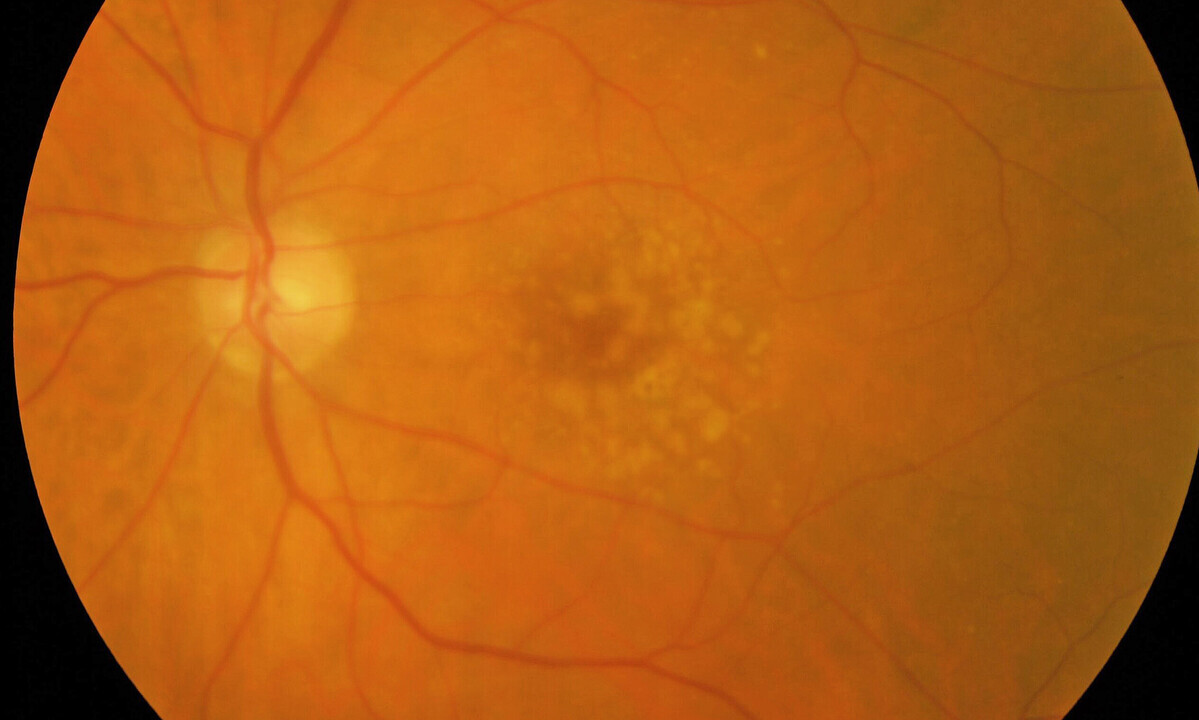
According to the researchers, the findings suggest drusen formation is a downstream effect of AKT2-related lysosome dysfunction and points to a new target for therapeutic intervention.
Drusen formation is still largely a mystery
Lysosomes are like cells' garbage disposals, and they play a crucial role in maintaining the retina. Key cells that make up the retinal pigment epithelium (RPE) provide oxygen and nutrients to the retina's energetically active neurons. They also collect and processes the retina’s waste products through lysosomes. Failure in the cells’ ability to process these waste products leads to the formation of drusen. As AMD progresses, drusen increase in number and volume. But despite intensive research, drusen formation is still largely a mystery.
In mice, the researchers manipulated AKT2 expression levels in RPE. When they overexpressed AKT2, lysosomes lost normal function and the mice developed dry AMD symptoms such as RPE degeneration. The researchers saw similar features in RPE cells from human donors with AMD or in RPE cells generated from patient stem cells. Cells from donors who possessed a gene variant called CFH Y402H, which increases AMD risk, had relatively greater expression of AKT2, showed functionally defective lysosomes, and formed drusen deposits.
Basis for a possible future treatment for dry AMD
This study's findings form the basis for a possible future treatment for dry AMD, for which no therapy currently exists. People with dry AMD develop drusen in an area of the light-sensing retina called the macula that people use for sharp, central vision.
The study builds upon previous work published by Ruchi Sharma, Ph.D., also of the NEI Section on Ocular Stem Cell and Translational Research Section, who developed the AMD patient stem cell-derived RPE model (Sharma et al., 2021).
Source: NHI
Articles: Ghosh S, Sharma R, et al. The AKT2/SIRT5/TFEB pathway as a potential therapeutic target in non-neovascular AMD. Nat Commun. 2024 Jul 21;15(1):6150. doi: 10.1038/s41467-024-50500-z
Sharma R, et al. Epithelial phenotype restoring drugs suppress macular degeneration phenotypes in an iPSC model. Nat Commun. 2021 Dec 15;12(1):7293. doi: 10.1038/s41467-021-27488-x