Communication between retinal cells: Swiss scientists solve a 50-year-old mystery
In a groundbreaking study published in Neuron, scientists at the Institute of Molecular and Clinical Ophthalmology Basel (IOB) have identified a surprisingly simple and novel mechanism that regulates how our eyes process visual information at the very first step of seeing. The study represents a significant leap forward in vision research and neuroscience.
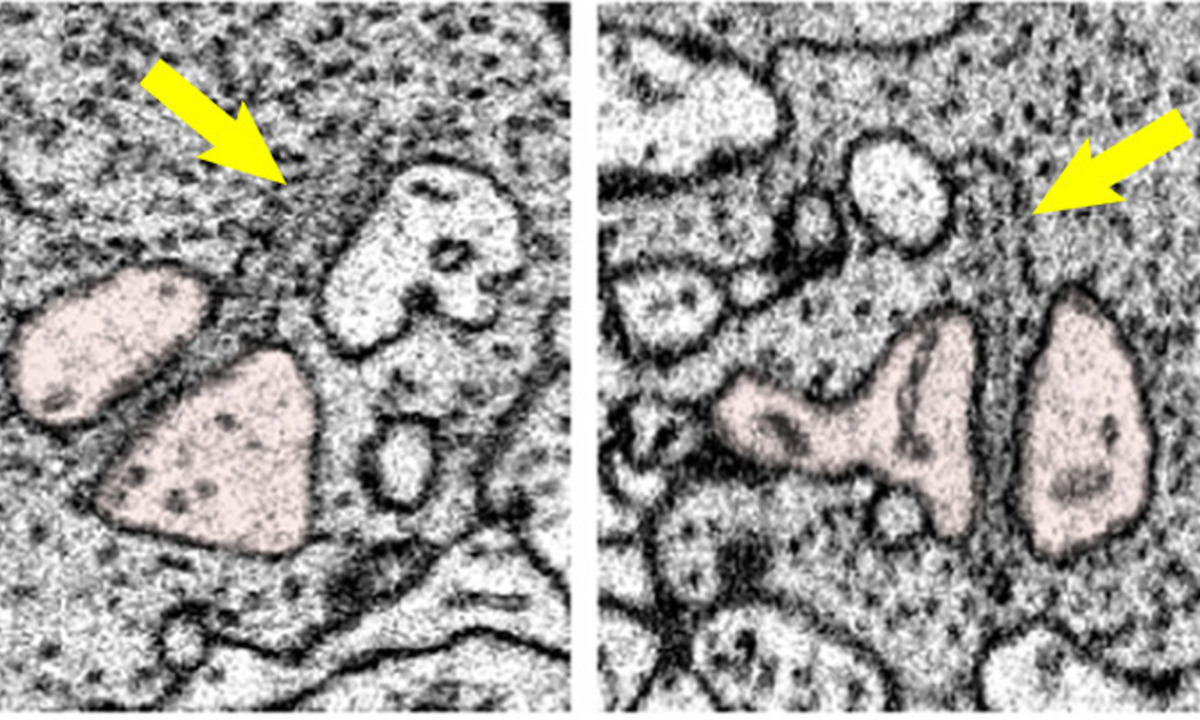
The study focuses on the intricate communication between two types of cells in the retina: photoreceptors (specifically cones) and horizontal cells. This interaction occurs at the first synapse of vision and plays a vital role in how we perceive the world around us.
Horizontal cells provide feedback to photoreceptors
"We have known for 50 years that horizontal cells provide feedback to photoreceptors, which is essential for various aspects of vision, including our ability to adapt to different light conditions and perceive contrasts," says Rei Morikawa, lead author of the study. "What is astonishing is the elegant simplicity and uniqueness of the mechanism we have uncovered."
The research team discovered that a protein called Slc4a5, found specifically in horizontal cells, is crucial for this feedback process. Slc4a5 is an electrogenic bicarbonate transporter, which means it can move bicarbonate ions across the cell membrane in response to changes in the cell's electrical state.
Unconventional and remarkably simple mechanism of feedback
"Our findings reveal an unconventional and remarkably simple mechanism of feedback at this critical juncture in the visual system," explains Botond Roska, senior author of the study. "When light hits the retina and changes the electrical state of horizontal cells, Slc4a5 modulates the transport of bicarbonate. This, in turn, alters the acidity levels in the tiny space between the cells, which ultimately affects how the photoreceptors respond to light."
What makes this discovery particularly intriguing is the simplicity of the system. The entire feedback mechanism relies on just two proteins: the Slc4a5 transporter in horizontal cells and a voltage-gated calcium channel in cone cells. This is in stark contrast to most other inhibitory neurons in the brain, which typically use a more complex system involving many proteins to transmit signals.
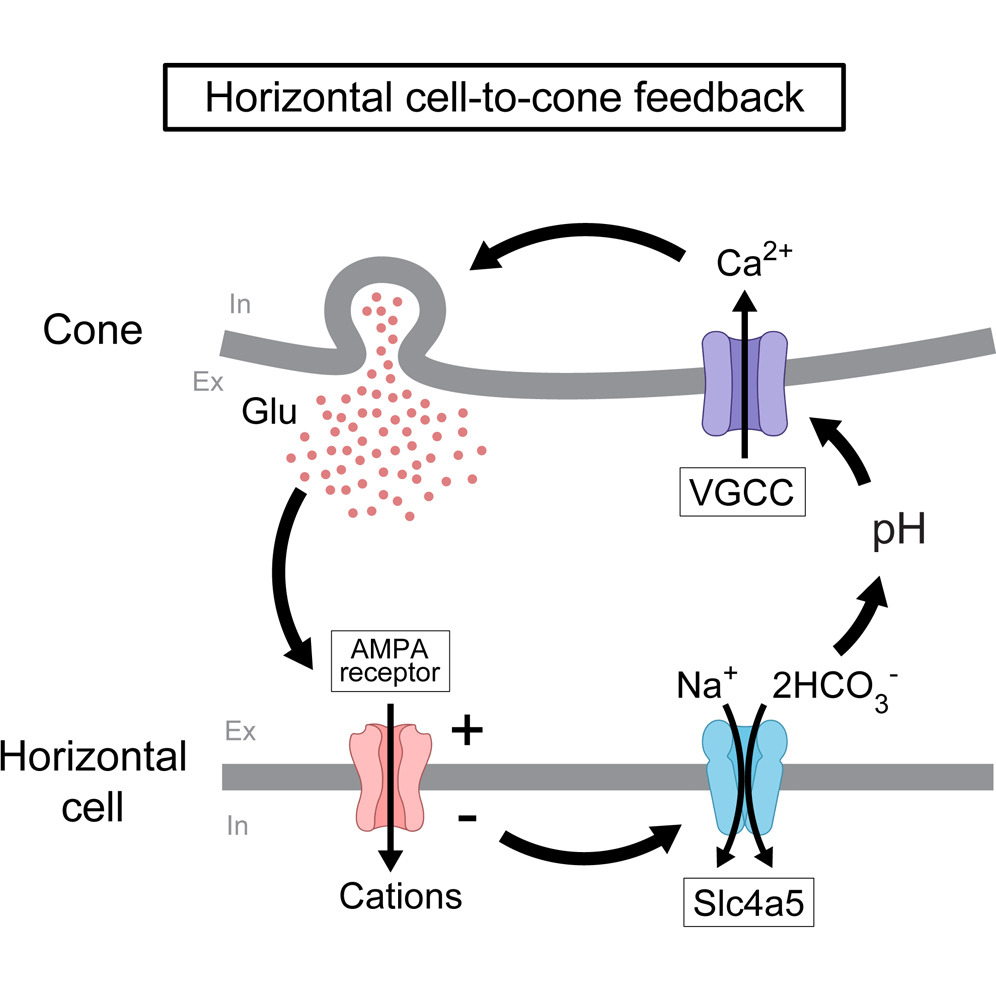
Graphical abstract. Image: Neuron
"It is fascinating to consider why evolution has favored such a streamlined approach at this crucial point in visual processing," Rei Morikawa adds. "This two-protein system may offer advantages in terms of speed or efficiency that are particularly well-suited to the demands of visual perception."
The team used a combination of advanced techniques to reach these conclusions: They created genetically modified mice lacking the Slc4a5 protein and also eliminated the same protein specifically in horizontal cells in adult mice. They employed two-photon imaging to observe the activity of many cone synapses simultaneously in the living retina. Finally, they used pharmacological tools to manipulate various aspects of cell signaling and ion transport.
Similar systems could exist elsewhere in the brain
This discovery has far-reaching implications for our understanding of neural communication and evolution.
"What we have found here might not be unique to the retina," Botond Roska commented. "The simplicity of this feedback mechanism suggests that similar systems could exist elsewhere in the brain. We are thrilled to explore whether other neural circuits might use comparable strategies for rapid, efficient signaling."
The research also raises intriguing questions about the evolution of neural communication: Why did this particular synapse evolve to use such a simple mechanism while other inhibitory neurons in the brain use more complex systems? The answer may lie in the specific requirements of visual processing at the first synapse, where specific speed and efficiency are required.
The study represents a significant leap forward in vision research and neuroscience. It not only solves a long-standing mystery but also opens up new avenues for understanding the evolution and optimization of neural circuits.
Source: IOB
Rei Morikawa, Tiago M. Rodrigues, Helene Marianne Schreyer, Cameron S. Cowan, Sarah Nadeau, Alexandra Graff-Meyer, Claudia P. Patino-Alvarez, Mohammad Hossein Khani, Josephine Jüttner, Botond Roska, The sodium-bicarbonate cotransporter Slc4a5 mediates feedback at the first synapse of vision, Neuron, 2024,ISSN 0896-6273,
https://doi.org/10.1016/j.neuron.2024.08.015.