AMD and Related Macular Dystrophies: Discovery of Novel Therapeutic Targets
New research published in the journal Developmental Cell provides important insights into the cellular mechanisms behind AMD and offers potential avenues for new treatments.
"Current treatments for AMD have limited efficacy and often come with significant side effects," said Ruchira Singh, PhD, with the University of Rochester Flaum Eye Institute and Center for Visual Sciences, and lead author of the study. "Our research aims to identify novel therapeutic targets that could potentially halt the progression of this disease."
Human stem cells to model AMD
The study utilized human stem cells to model AMD, overcoming the limitations of previous research using animal models. By examining genes associated with both AMD and and related macular dystrophies (MDs), such as Sorsby’s fundus dystrophy, Doyne honeycomb macular dystrophy, and autosomal dominant radial drusen, the researchers identified a key protein involved in the early stages of the disease.
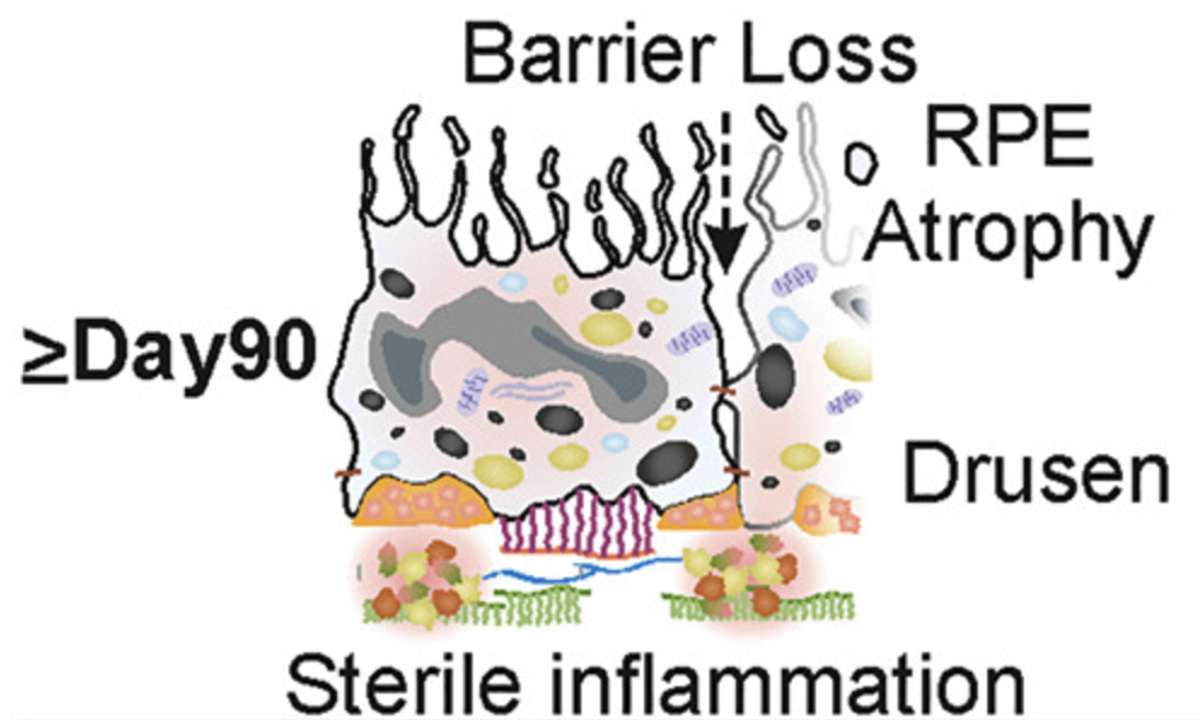
The retinal pigment epithelium (RPE) plays a crucial role in AMD. Over time, deposits of lipids and proteins, known as drusen, accumulate in the RPE. These deposits are often an early indicator of AMD.
The researchers discovered that a protein called tissue inhibitor of metalloproteinases 3 (TIMP3) is overproduced in AMD. TIMP3 inhibits the activity of enzymes called matrix metalloproteinases (MMPs), which are essential for eye health. Impaired MMP activity leads to increase in another enzyme which promotes inflammation and the formation of drusen.
Reduction of drusen formation
By using a small molecule inhibitor to block the activity of the enzyme associated with inflammation, the researchers were able to reduce drusen formation in their model, suggesting that targeting this pathway could be a promising strategy for preventing AMD.
"Cellular pathways involved in drusen formation are key drivers of AMD progression," said Dr. Singh. "If we can halt the accumulation of drusen, we may be able to prevent the disease from progressing to a stage where vision loss occurs. This research offers hope for developing new treatments that could significantly improve the lives of millions of people affected by AMD."
The take-aways from the study are as follows according to the authors:
- Identification of a shared mechanistic defect in AMD and MDs
- Reduced activity of RPE-secreted MMP2 instigates pro-maculopathy cellular events
- Perturbation of the MMP2-DAMP-RAGE-sPLA2-IIA axis contributes to AMD/MD pathology
- Pharmacologic targeting in a human iPSC model of AMD and 3 distinct MDs
Source: University of Rochester
Dalvi S, Roll M, Chatterjee A, et al. Human iPSC-based disease modeling studies identify a common mechanistic defect and potential therapies for AMD and related macular dystrophies. Dev Cell. 2024; https://doi.org/10.1016/j.devcel.2024.09.006